Solubility O2 in H2O
How much oxygen (O2) is soluble in water (H2O)?
With fine bubble aeration 9 mg O2 per liter water can be dissolved, with a water temperature of 20 degrees Celsius. The water temperature determines how much O2 can be solved as shown in the following table:
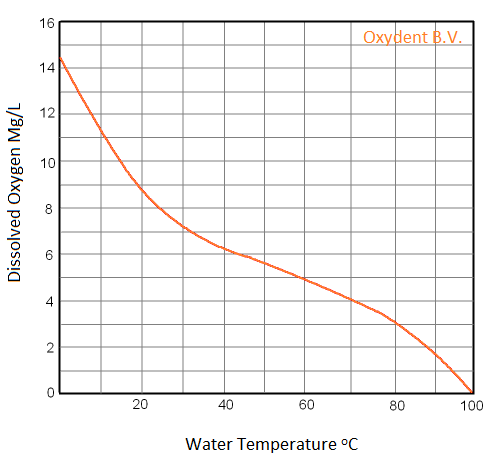
Higher O2 mg / l than in the table?
Outside air roughly consists of 20% oxygen and 78% nitrogen and 2% other gases. If one would use pure oxygen and take out the other gases, then the saturation is about 45 mg O2 per liter water.

 Deutsch
Deutsch
 English
English
 Français
Français
 Nederlands
Nederlands